X-ray structure of two Schiff bases: TURN-ON sensing of Fe3+ and Al3+ in the HepG2 cell line†
Abstract
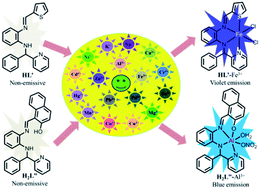
The efficiency of the fluorescence sensitivity of a sensor may be tuned by the modulation of the steric and electronic parameters in the structure. In this study, the thiophenyl Schiff base (E)-N1-(phenyl(pyridin-2-yl)methyl)-N2-(thiophen-2-ylmethylene)benzene-1,2-diamine (HL′) exhibited very high selectivity and a sensitive fluorescence enhancement towards Fe3+ with violet emission (λem, 385 nm; LOD, 3.8 nM). On the other hand, the naphthyl Schiff base (E)-1-(((2-((phenyl(pyridin-2-yl)methyl)amino)phenyl)imino)methyl)naphthalen-2-ol (H2L′′) exhibited fluorescence sensitivity towards Al3+, showing blue emission (λem, 502 nm; LOD, 3.3 nM) in H2O (HEPES buffer, pH 7.4) medium. The emission enhancement of HL′ upon binding to Fe3+ may be considered to be due to the restriction of intramolecular rotation, while the selectivity of H2L′′ towards Al3+ may be due to the turn on emission through the restriction of excited state intramolecular proton transfer (ESIPT) and the introduction of chelation enhanced fluorescence (CHEF). Furthermore, DFT computation supported the sensing strategy and the probes were applied for intracellular detection of Fe3+ and Al3+ in HepG2 cell lines.


 Please wait while we load your content...
Please wait while we load your content...