Development of a universal method for the measurement of binding affinities of antibody drugs towards a living cell based on AFM force spectroscopy
Abstract
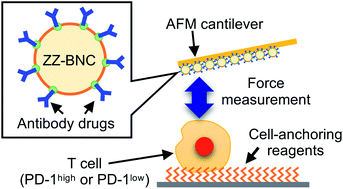
A universal method to measure the binding affinities of antibody drugs towards their targets on the surface of living cells was developed based on atomic force microscopy (AFM) analysis. Nivolumab, an antibody drug targeting programmed cell death 1 (PD-1), was mainly used as a model for this evaluation. The surface of a tip-less AFM cantilever was coated with nano-capsules, on which immunoglobulin G-binding ZZ domains of protein A were exposed, and nivolumab molecules were immobilized on the cantilever through binding between the antibody Fc domains and the ZZ domains, which controlled the molecular orientation of the antibodies. Model human T lymphocytes (Jurkat), on which PD-1 molecules were highly expressed, were immobilized on a glass substrate via a lipid bilayer-anchoring reagent. The nivolumab-coated AFM cantilever was moved to approach the T cells, and the rupture forces between nivolumab molecules on the AFM cantilever and PD-1 molecules on the cell surface were measured. The average values of the rupture forces were 0.18 ± 0.10, 0.21 ± 0.18, 0.12 ± 0.07, 0.11 ± 0.06, and 0.12 ± 0.06 nN μm−2 at loading forces of 10, 20, 30, 40, and 50 nN, respectively. Application of significantly higher loading forces decreased the S/N ratio, as confirmed by comparison with control T cells with low PD-1 expression, which suggested that a low loading force of less than 20 nN was sufficient for these measurements. A correlation between the expression levels of PD-1 and the rupture force values was confirmed using immunofluorescence. A similar assay was performed by using an antibody drug targeting epidermal growth factor receptor (EGFR) and a model cancer cell expressing EGFR molecules (A431) to evaluate the universal application of the developed method for various antibody drugs, and the same conclusions as that in nivolumab's case were obtained. This method can be applied to living cells without any chemical treatment, which allows the present method to compare the affinities of various antibody drugs towards the same single cell. These results indicated that the present method is useful for selecting the most effective candidates from various antibody drugs from the point of view of binding forces between antibodies and living cells.


 Please wait while we load your content...
Please wait while we load your content...