Electrochemical nucleation and growth of Pt nanoflower particles on reduced graphite oxide with electrooxidation of glucose†
Abstract
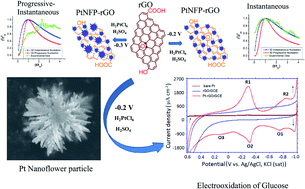
Electrochemical deposition of platinum nanoflower particles (PtNFPs) on reduced graphite oxide, rGO/GCE, using H2PtCl6 electrolyte in H2SO4 solution was investigated by chronoamperometry (CA). The experimental i–t curves measured at different overpotentials were analyzed and compared with theoretical curves obtained for the two limiting cases of the 3D nucleation and growth model, as described by Scharifker and Hills. The effect of deposition overpotential on the deposition process was evaluated. Through comparing potentiostatic current–time transients with the Scharifker–Hills model, a transition from instantaneous to a mixed kind of progressive–instantaneous nucleation was observed when increasing the deposition overpotential. CA results clearly show that the electrodeposition processes are diffusion-controlled (D0 = 2.77 × 10−5 cm2 s−1). A finer distribution of PtNFPs with an average size of ∼70 nm was obtained in 1.0 mM H2PtCl6 electrolyte in a 0.5 M H2SO4 solution. The as-prepared PtNFP/rGO catalyst shows 1.5 times higher activity than rGO/GCE, and the catalytic activity towards glucose oxidation is about many fold higher than that of the rGO/GCE and bare platinum electrode.


 Please wait while we load your content...
Please wait while we load your content...