A novel nanoplatform encapsulating glucose oxidase for spectrophotometric biosensing of hydrogen peroxide and glucose
Abstract
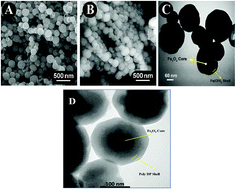
In this work, we were inspired from the role of chelation of Fe3+–catechol in inter-protein interactions and the production of adhesives by marine mussels, and used dopamine (DA) as an anchor to connect the enzyme glucose oxidase (GOx) to Fe3O4 magnetic nanoparticle cores via the formation of Fe(OH)3 shells. Because of the tendency of catechol and similar ligands such as DA to coordinate with the Fe3+ surface sites, a tight binding of DA to the Fe3O4–Fe(OH)3 core–shell was easily accomplished. Accordingly, we formulated an Fe3+–polyDA framework to encapsulate GOx; we specifically produced Fe3O4–Fe(OH)3@GOx–polyDA by carrying out an in situ polymerization of DA covalently linked to GOx on the Fe(OH)3 shells of magnetic nanoparticles. The Fe3+–polyDA framework stabilized the structure of the encapsulated GOx layer and increased its thermal stability, operational stability and recyclability, while preserving its activity. The prepared Fe3O4–Fe(OH)3@GOx–polyDA probe displaying enzyme-like characteristics was used as a multifunctional platform in a sensitive and selective spectrophotometric biosensor, with N,N-diethyl-p-phenylenediamine sulfate (DPD) as a redox indicator, for sub-micromolar detection of hydrogen peroxide and glucose via an enzymatic cascade reaction.


 Please wait while we load your content...
Please wait while we load your content...