Comparative effects of surfactants on the behavior of an anticancer drug potentiometric sensor
Abstract
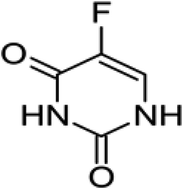
Surfactants may incorporate drug molecules in a micelle, allowing a great enhancement of a 5-fluorouracil electrode. The performance characteristics of electrodes comprising selected surfactants were investigated as well as the effect of their concentrations. These surfactants were benzalkonium chloride as a cationic surfactant, sodium dodecylsulfate as an anionic surfactant and Tween 20 and Tween 80 as non-ionic surfactants. In solutions of 5-fluorouracil in benzalkonium chloride the sensor showed the best response with a Nernstian slope of 29.5 mV per decade, a wide concentration range from 5.4 × 10−6 to 1.0 × 10−2 M, a notably low detection limit of 4.4 × 10−6 M and a significantly short response time (5 s). The electrode response is independent of the pH of the test solution in the range 3.2–9.5. Selectivity toward the drug ion was calculated for different inorganic cations, amino acids and pharmaceutical amines. Direct potentiometry and potentiometric titration were applied for the determination of 5-fluorouracil in its pure form and as an ampoule formulation and in urine.


 Please wait while we load your content...
Please wait while we load your content...