Generating linear oxygen gradients across 3D cell cultures with block-layered oxygen controlled chips (BLOCCs)†
Abstract
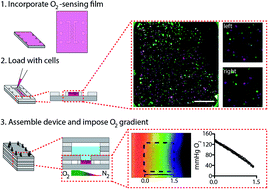
Oxygen is a transcriptional regulator responsible for tissue homeostasis and maintenance. Studies relating cellular phenotype with oxygen tension often use hypoxia chambers, which expose cells to a single, static oxygen tension. Despite their ease of use, these chambers are unable to replicate the oxygen gradients found in healthy and diseased tissues. Microfabricated devices capable of imposing an oxygen gradient across tissue-like structures are a promising tool for these studies, as they can provide a high density of information in a single experimental setup. We describe the fabrication and characterization of a modular device, which leverages the gas-permeability of silicone to impose gradients of oxygen across cell-containing regions, assembled by layering sheets of laser cut acrylic and silicone rubber. The silicone also acts as a barrier, separating the flowing gases from the cell culture medium, preventing evaporation or bubble formation in experiments that require prolonged periods of incubation. The acrylic components provide a rigid framework to provide a sterile culture environment. Using oxygen-sensing films, we show the device can support gradients of different ranges and steepness by simply changing the composition of the gases flowing through the silicone components of the BLOCC. Using a cell-based reporter assay, we demonstrate that cellular responses to hypoxia are proportional to oxygen tension.
- This article is part of the themed collection: Bioanalytical sensors for real world applications


 Please wait while we load your content...
Please wait while we load your content...