A one-step molded microfluidic chip featuring a two-layer silver-PDMS microelectrode for dielectrophoretic cell separation†
Abstract
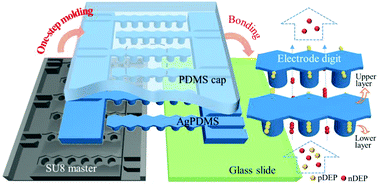
Dielectrophoresis (DEP) is a powerful technique for label-free cell separation in microfluidics. Easily-fabricated DEP separators with low cost and short turnaround time are in extremely high demand in practical applications, especially clinical usage where disposable devices are needed. DEP separators exploiting microelectrodes made of conducting polydimethylsiloxane (PDMS) composites enable the construction of advantageous 3D volumetric electrodes with a simple soft-lithography process. Yet, existing devices incorporating microelectrodes in conducting PDMS generally have their fluidic sidewalls constructed using a different material, and consequently require extra lithography of a sacrificial layer on the semi-finished master for molding the electrode and fluidic sidewalls in separate steps. Here we demonstrate a novel microfluidic DEP separator with a 3D electrode and fluidic structure entirely integrated within silver-PDMS composites. We develop a further simplified one-step molding process with lower cost using a readily-available and reusable SU8 master, eliminating the need for the additional lithography step in existing techniques. The uniquely designed two-layer electrode exhibits a spatially non-uniform electric field that enables cell migration in the vertical direction. The electrode upper layer then offers a harbor-like region for the trapping of the target cells that have drifted upwards, which shelters them from being dragged away by the main flow streams in the lower layer, and thus allows higher operation flow rate. We also optimize the upper layer thickness as a critical dimension for protecting the trapped cells from high drag and show easy widening of our device by elongation of the digits. We demonstrate that the elongated digits involving more parallel flow paths maintain a high capture efficiency of 95.4% for live cells with 85.6% purity in the separation of live/dead HeLa cells. We also investigate the device feasibility in a viability assay for cells post anti-cancer drug treatment.

 Please wait while we load your content...
Please wait while we load your content...