Two-dimensional identification and localization of isomers in crystallin peptides using TWIM-MS†
Abstract
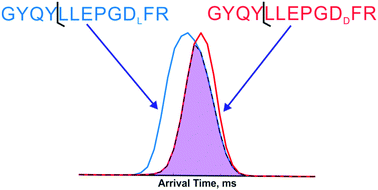
Recent studies have illuminated connections between spontaneous chemical reactions that cause isomerization at specific protein residues and various age-related diseases including cataracts and Alzheimer's. These discoveries provide impetus for better analytical methods to detect and characterize isomerization in proteins, which will enable a more complete understanding of the underlying relationship between these modifications and biology. Herein we employ a two-dimensional approach for identification of peptides isomers that also includes pinpointing of the modified residue. Collision-induced dissociation is used to fragment ions in the first dimension, followed by separation of the fragments with travelling-wave ion mobility. By comparing data obtained from both isomers, differences in either fragment-ion intensities or arrival-time distributions can be used to identify isomeric forms and the specific site of modification within the peptides. Synthetic peptide standards with sequences derived from long-lived proteins in the eye lens and isomerization at serine, aspartic acid, and glutamic acid were examined. Although both dimensions are capable of isomer identification, ion mobility is much better at determining the site of modification. In general, separation of isomeric forms by ion mobility is possible but does not follow predictable trends dictated by sequence or fragment-ion length. In most cases, however, the site of isomerization can be precisely determined.


 Please wait while we load your content...
Please wait while we load your content...