An N-linked disalicylaldehyde together with its caesium ion and dichloromethane sensing performances: ‘dual key & lock’ LMCT-enhanced fluorescence strategy†
Abstract
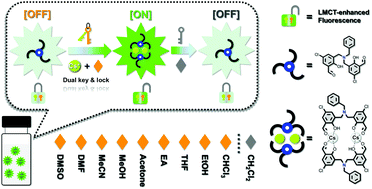
The development of inexpensive, selective and rapid-response chemosensors for detecting Cs+ in waste water is highly desirable in the nuclear power industry. Here we demonstrate an efficient Cs+ optical sensor based on the N-linked disalicylaldehyde H2Qj with excited state intramolecular proton transfer (ESIPT), and it will transform into the ligand-to-metal charge transfer (LMCT) process in the presence of Cs+, resulting in dramatically enhanced fluorescence together with a distinct change of color from light-green to green-yellow. Simultaneously, it is found that CH2Cl2 can serve as the quencher of LMCT-enhanced fluorescence, thus enabling selective CH2Cl2 detection in a turn-off fluorescence approach. Further detailed studies reveal that both Cs+ and CH2Cl2 sensing processes are rapid within 60 seconds. The corresponding limit of detection (LOD) values for sensing Cs+ and CH2Cl2 are as low as 0.37 mM and 0.37%. Moreover, it was also verified that Cs+ sensing is applicable in the range of pH = 7–11 and the reversibility of sensor H2Qj can be easily achieved by modulating pH values, and H2Qj is also assessed for its Cs+ sensing performces in real water samples. This H2Qj-Cs sensing system must provide a valuable reference for further Cs+ sensors.


 Please wait while we load your content...
Please wait while we load your content...