Dynamic behavior analysis of ion transport through a bilayer lipid membrane by an electrochemical method combined with fluorometry†
Abstract
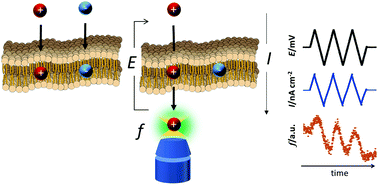
To examine the transport of an ionic substance through a bilayer lipid membrane (BLM), an electrochemical method combined with fluorometry was proposed. In this method, the transport of a fluorescent ion through the BLM was detected both as the transmembrane current and the dynamic change of fluorescence intensity synchronizing scanning membrane potential. The fluorescence intensity was measured in the local area close to the planar BLM by utilizing a confocal fluorescence microscope. The electrochemical method combined with fluorometry makes it possible to analyze only the transport of a target fluorescent ion in distinction from the transport of other coexisting ions. With the proposed electrochemical method, the ion transport caused by both a hydrophobic fluorescent cation (rhodamine 6G+, R6G+) and a relatively hydrophobic anion (BF4−) was examined. The electrochemical method combined with fluorometry characterized the transmembrane current as the transport of R6G+. Membrane conductance for the R6G+ transport increased proportionally to the concentrations of R6G+ and BF4− distributed in the hydrocarbon medium of the BLM which were estimated by extraction experiments with liposomes. These results show that the distribution of a cation and an anion from the aqueous phase in the BLM predominantly controls the membrane conductance for ion transport through the BLM.


 Please wait while we load your content...
Please wait while we load your content...