Mechanistic insights into heavy metal ion sensing by NOS2-macrocyclic fluorosensors via the structure-function relationship: influences of fluorophores, solvents and anions†
Abstract
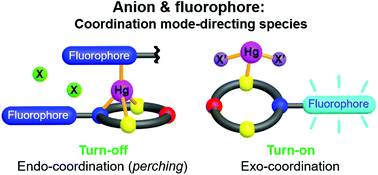
To obtain a mechanistic understanding of the effects derived from fluorophores, solvents and anions on heavy metal sensing, two NOS2-macrocycle-based fluorosensors with different fluorophores (L1: 9-methylanthracene, L2: benzothiazolyl) were synthesised. In this regard, particular attention was given to monitoring the cation–ligand, cation–anion and cation–solvent interactions from the detailed complexation processes in both the solution and solid states while considering the structure-function relationship. L1 showed turn-on type silver(I) selectivity over other metal ions, including mercury(II), in ethanol. According to the complexation results obtained by titration (UV-vis, fluorescence and NMR), cold-spray ionization mass spectrometry and X-ray crystallography, the observed silver(I) sensing by L1 is mainly due to its 1 : 1 complexation with silver(I) via the Ag–Ntert bond and the strong solvation of mercury(II). Thus, the turn-on sensing for silver(I) can be explained by the CHEF effect, in which the coordination of silver(I) to the receptor unit effectively prevents PET quenching. As a dual-probe (UV-vis and fluorescence) chemosensor, L2 showed fluorescence turn-off type selectivity for both silver(I) and mercury(II) in ethanol. In acetonitrile, L2 showed improved fluorescence turn-off type selectivity for mercury(II) with ClO4− and NO3−; however, no such responses were observed with other anions, such as Cl−, Br−, I−, SCN−, OAc− and SO42−. Together with the complexation results by titration, the crystal structures of an endocyclic mercury(II) perchlorate complex and an exocyclic mercury(II) iodide complex revealed that the anion-controlled mercury(II) sensing by L2 arises from the endo- and exo-coordination modes depending on the anion coordinating ability, which induces either metal–receptor/fluorophore binding (Hg–Ntert and Hg–Nfl) or no binding. Taken collectively, the photophysical, thermodynamic and structural results of the complexations herein suggest that the sensing properties of heavy metal ions by macrocycle-based fluorosensors are very sensitive not only to the cation–receptor and cation–fluorophore interactions but also to the cation–anion (endo/exo-coordination modes) and/or cation–solvent interactions.


 Please wait while we load your content...
Please wait while we load your content...