A bis-indole/carbazole based C5-curcuminoid fluorescent probe with large Stokes shift for selective detection of biothiols and application to live cell imaging†
Abstract
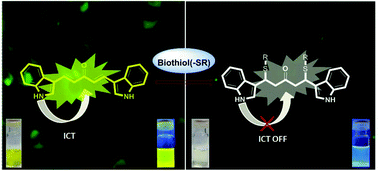
A series of heterocyclic C5-curcuminoids (bis(arylmethylidene)acetones) (PJ1–PJ6) having a large Stokes shift (Δλ = 104–173 nm) have been synthesized for the selective detection of cysteine (Cys), homocysteine (Hcy) and glutathione (GSH) in living cells. The compounds were synthesized using a new methodology via deacetylation under microwave conditions. The photophysical properties of these compounds have been studied. Prominent colour changes from bright yellow to colourless in the presence of thiols were observed for PJ1. Live cell imaging has been employed with PJ1 for the utilization of the probe to detect homocysteine in A375 cells and apoptosis in AGS cells.


 Please wait while we load your content...
Please wait while we load your content...