Current developments in LC-MS for pharmaceutical analysis
Abstract
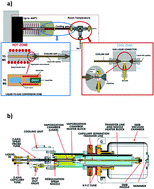
Liquid chromatography (LC) based techniques in combination with mass spectrometry (MS) detection have had a large impact on the development of new pharmaceuticals in the past decades. Continuous improvements in mass spectrometry and interface technologies, combined with advanced liquid chromatographic techniques for high-throughput qualitative and quantitative analysis, have resulted in a wider scope of applications in the pharmaceutical field. LC-MS tools are increasingly used to analyze pharmaceuticals across a variety of stages in their discovery and development. These stages include drug discovery, product characterization, metabolism studies (in vitro and in vivo) and the identification of impurities and degradation products. The increase in LC-MS applications has been enormous, with retention times and molecular weights (and related fragmentation patterns) emerging as crucial analytical features in the drug development process. The goal of this review is to give an overview of the main developments in LC-MS based techniques for the analysis of small pharmaceutical molecules in the last decade and give a perspective on future trends in LC-MS in the pharmaceutical field.


 Please wait while we load your content...
Please wait while we load your content...