Characterization of the furin cleavage motif for HIV-1 trimeric envelope glycoprotein by intact LC-MS analysis†
Abstract
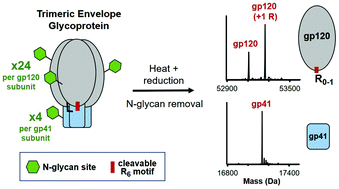
Generating a soluble and native-like trimeric envelope glycoprotein (Env) with high efficacy as an immunogen has been a major focus for developing an effective vaccine against HIV-1. The Env immunogen is a heavily glycosylated protein composed of 3 identical surface gp120 and gp41 subunits that form into a trimer of heterodimers (3 × 28 N-glycan sites). During Env immunogen production, endogenous furin works to cleave a hexa-arginine motif connecting the gp120 and gp41 subunits, which is needed to ensure proper protein folding and a native-like conformation of Env. Verification of the overall identity and proteolytic cleavage of Env is therefore important for HIV-1 vaccine development and product quality. Herein, we report the first work using LC-MS to (1) achieve fast and accurate intact mass measurement of Env after deglycosylation and (2) confidently identify the furin cleavage sites.


 Please wait while we load your content...
Please wait while we load your content...