Rational design of a novel turn-on fluorescent probe for the detection and bioimaging of hydrazine with barbituric acid as a recognition group †
Abstract
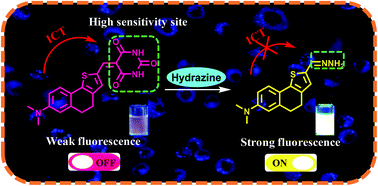
A novel turn-on fluorescent probe with barbituric acid as a unique recognition group has been rationally designed and synthesized using a facile method for detecting hydrazine. The 5-((7-(dimethylamino)-4,5-dihydronaphtho [1,2-b] thiophen-2-yl)methylene)pyrimidine-2,4,6 (1H,3H,5H)-trione (DPT) probe displays a large emission signal ratio variation (more than a 40-fold enhancement) in the presence of hydrazine under neutral conditions. Interestingly, a novel recognition mechanism based on a hydrazine-triggered addition–cyclisation-retro aldol was proposed and confirmed. Additionally, the DPT probe exhibits a low detection limit (5 × 10−8 M), applicable to the physiological pH range (3–12), a broad linear response range for hydrazine concentrations between 0 and 34 μM and a large Stokes shift (147 nm) for hydrazine detection in aqueous solution. Moreover, the DPT probe was successfully implemented for hydrazine imaging in vivo.


 Please wait while we load your content...
Please wait while we load your content...