Ring fusion in tetrathienylethene cored perylene diimide tetramers affords acceptors with strong and broad absorption in the near-UV to visible region†
Abstract
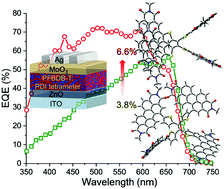
In this work, we designed and synthesized two novel perylene diimide (PDI) tetramers based on a tetrathienylethene core, named TTE-PDI4 and FTTE-PDI4, and investigated their application as non-fullerene acceptors for organic photovoltaics. The free rotation of PDIs and adjacent thiophene units renders TTE-PDI4 with a highly twisted molecular geometry. The ring fusion of TTE-PDI4 yields FTTE-PDI4, a more rigid molecule with increased intramolecular stacking. Interestingly, TTE-PDI4 and FTTE-PDI4 possess similar energy levels but very different UV-Vis absorptions, with the latter showing strong broad-band absorption with multiple sharp peaks in the 300–600 nm region. Through time-dependent density functional theory (TD-DFT) calculations, we show that this broad absorption spectrum in FTTE-PDI4 arises from the combination of multiple bright transitions in the visible region with a strong vibronic progression, tentatively assigned to the dominant C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching mode. TTE-PDI4, despite having a lower energy absorption onset, shows weaker absorption at long wavelengths. Due to its higher absorption as well as its increased rigidity, FTTE-PDI4 shows a higher photocurrent and hence a higher power conversion efficiency (PCE), of 6.6%, when blended with the polymer donor PFBDB-T than TTE-PDI4 based blends (PCE of 3.8%). The greater rigidity of FTTE-PDI4 is likely to contribute to the good fill factor of the blend devices. Potential for further improvement through reducing voltage losses is identified.
C stretching mode. TTE-PDI4, despite having a lower energy absorption onset, shows weaker absorption at long wavelengths. Due to its higher absorption as well as its increased rigidity, FTTE-PDI4 shows a higher photocurrent and hence a higher power conversion efficiency (PCE), of 6.6%, when blended with the polymer donor PFBDB-T than TTE-PDI4 based blends (PCE of 3.8%). The greater rigidity of FTTE-PDI4 is likely to contribute to the good fill factor of the blend devices. Potential for further improvement through reducing voltage losses is identified.
- This article is part of the themed collections: Celebrating our 2024 Prizewinners and Celebrating Tobin Marks’ 75th Birthday


 Please wait while we load your content...
Please wait while we load your content...