Synthesis and photoswitchable amphiphilicity and self-assembly properties of photochromic spiropyran derivatives†
Abstract
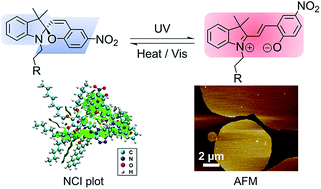
A new class of amphiphilic spiropyran derivatives has been designed and synthesized. Their photophysical and photochromic properties have been investigated. Under UV light irradiation, the ring-closed hydrophobic spiropyrans have been shown to undergo photoinduced ring-opening to give the zwitterionic ring-opened merocyanine forms, which resulted in the amphiphilic properties of the compounds. These compounds were also found to display self-assembly behavior with the formation of H-aggregation in organic solvents under UV irradiation to give different morphologies with diverse nano-structures, leading to promising candidates for the design of a new class of small-molecule photo-responsive materials. The H-aggregate formation has been further supported by computational studies and noncovalent interaction (NCI) analysis of the dimer of the merocyanine form. This represents the first demonstration of the use of NCI analysis on the role played by noncovalent interactions in H-aggregate formation of the spiropyran derivatives.
- This article is part of the themed collection: Editor’s Choice: Malika Jeffries-EL


 Please wait while we load your content...
Please wait while we load your content...