Liquid phase exfoliation of MoS2 and WS2 in aqueous ammonia and their application in highly efficient organic solar cells†
Abstract
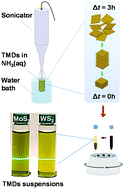
Simple, scalable and cost-effective synthesis of quality two-dimensional (2D) transition metal dichalcogenides (TMDs) is critical for fundamental investigations but also for the widespread adoption of these low-dimensional materials in an expanding range of device applications. Here, we report on the liquid-phase exfoliation (LPE) of molybdenum disulfide (MoS2) and tungsten disulfide (WS2) in aqueous ammonia (NH3(aq.)) as a greener alternative to commonly used but less environmentally friendly solvents. The synthesized nanosheets can be prepared in high concentrations (0.5–1 mg mL−1) and exhibit excellent stoichiometric and structural quality with a semiconducting character. These characteristics make them ideal for application in organic optoelectronics, where optical transparency and suitable energetics are two important prerequisites. When MoS2 and WS2 are used as the hole transport layer materials in organic photovoltaics, cells with a power conversion efficiency of 14.9 and 15.6%, respectively, are obtained, highlighting the potential of the aqueous ammonia-based LPE method for the preparation of high quality TMDs. The method could potentially be extended to other TMDs.
- This article is part of the themed collection: 2020 Journal of Materials Chemistry C most popular articles


 Please wait while we load your content...
Please wait while we load your content...