Red luminescent Eu2+ in K2MgH4 and comparison with KMgH3†
Abstract
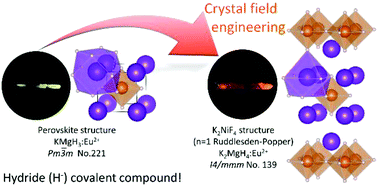
The engineering of covalency and crystal field is a key point for the control of the 5d energy level of Eu2+. The hydride ion exhibits strong covalency, which results in a large 5d energy shift by the nephelauxetic effect compared to their fluoride analogues. In this study, the Eu2+-doped K2MgH4 Ruddlesden–Popper (n = 1) perovskite was prepared and its optical properties were investigated. Red luminescence due to the 4f65d1 → 4f7 transition was observed at 670 nm by UV excitation of the K2MgH4:Eu2+ sample. Compared with yellow Eu2+-luminescence at 565 nm of the KMgH3 cubic perovskite, which has almost the same chemical composition as K2MgH4, the additional red-shift of the luminescence in K2MgH4:Eu2+ is caused by the strong crystal field splitting in the unique 9-fold coordinated site in addition to the large nephelauxetic effect by hydride ions. Using a hydride model system that allows for a clear distinction between crystal field splitting and nephelauxetic effect, we successfully demonstrated the design concept for the 5d energy shifting by covalency and crystal field in Eu2+-doped K2MgH4. Also, the thermal quenching process of K2MgH4:Eu2+ by the ionization process was discussed in comparison to KMgH3:Eu2+ on the basis of the energy diagram.


 Please wait while we load your content...
Please wait while we load your content...