Utilizing Diels–Alder “click” chemistry to functionalize the organic–organic interface of semiconducting polymers†
Abstract
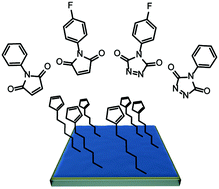
Tuning the energy levels of organic semiconductors in a predictable and durable manner is a challenging, yet necessary task to obtain high performance electronic and optoelectronic devices. Thermal diffusion and drift of dopants used for energy level control in the mostly van der Waals-cohered solids results in the degradation of the defined interfaces that all electronic and optoelectronic devices rely on. An important goal is therefore their spatial fixation in multilayer organic device stacks. This work aims at covalently functionalizing the interface of an immobilized organic semiconductor polymer. To achieve this goal, the side chains of a polyfluorene are equipped with biscyclopentadienyl moieties which undergo thermal fragmentation via retro-Diels–Alder reactions to yield cyclopentadienyl functions. Diels–Alder cycloadditions of these functional groups cross-link adjacent polymer chains. In a second step, unreacted cyclopentadienyl units at the interface are covalently functionalized with strong dienophiles through a Diels–Alder reaction from solution. This reaction sequence modifies the energetics and the work function at the interface. Transfer of our protocol to other conjugated polymers and/or reaction types paves the way to tunable energy levels at organic–organic interfaces and to universal introduction of functions at organic semiconductor interfaces after deposition from solution.


 Please wait while we load your content...
Please wait while we load your content...