Photoinduced reconfiguration to control the protein-binding affinity of azobenzene-cyclized peptides†
Abstract
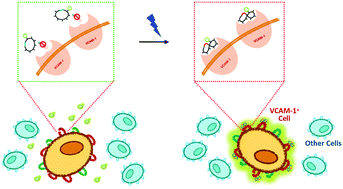
The impact of next-generation biorecognition elements (ligands) will be determined by the ability to remotely control their binding activity for a target biomolecule in complex environments. Compared to conventional mechanisms for regulating binding affinity (pH, ionic strength, or chaotropic agents), light provides higher accuracy and rapidity, and is particularly suited for labile targets. In this study, we demonstrate a general method to develop azobenzene-cyclized peptide ligands with light-controlled affinity for target proteins. Light triggers a cis/trans isomerization of the azobenzene, which results in a major structural rearrangement of the cyclic peptide from a non-binding to a binding configuration. Critical to this goal are the ability to achieve efficient photo-isomerization under low light dosage and the temporal stability of both cis and trans isomers. We demonstrated our method by designing photo-switchable peptides targeting vascular cell adhesion marker 1 (VCAM1), a cell marker implicated in stem cell function. Starting from a known VCAM1-binding linear peptide, an ensemble of azobenzene-cyclized variants with selective light-controlled binding were identified by combining in silico design with experimental characterization via spectroscopy and surface plasmon resonance. Variant cycloAZOB[G-VHAKQHRN-K] featured rapid, light-controlled binding of VCAM1 (KD,trans/KD,cis ∼ 130). Biotin-cycloAZOB[G-VHAKQHRN-K] was utilized to label brain microvascular endothelial cells (BMECs), showing co-localization with anti-VCAM1 antibodies in cis configuration and negligible binding in trans configuration.


 Please wait while we load your content...
Please wait while we load your content...
