Hexametaphosphate as a potential therapy for the dissolution and prevention of kidney stones†
Abstract
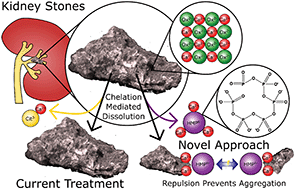
The incidence of kidney stones is increasing worldwide, and recurrence is common (50% within 5 years). Citrate, the current gold standard therapy, which is usually given as potassium or sodium salts, is used because it raises urine pH and chelates calcium, the primary component of up to 94% of stones. In this study hexametaphosphate (HMP), a potent calcium chelator, was found to be 12 times more effective at dissolving calcium oxalate, the primary component of kidney stones, than citrate. HMP was also observed to be effective against other common kidney stone components, namely calcium phosphate and struvite (magnesium ammonium phosphate). Interestingly, HMP was capable of raising the zeta potential of calcium oxalate particles from −15.4 to −34.6 mV, which may prevent stone growth by aggregation, the most rapid growth mechanism, and thus avert occlusion. Notably, HMP was shown to be up to 16 times as effective as citrate at dissolving human kidney stones under simulated physiological conditions. It may thus be concluded that HMP is a promising potential therapy for calcium and struvite kidney stones.
- This article is part of the themed collection: Journal of Materials Chemistry B HOT Papers


 Please wait while we load your content...
Please wait while we load your content...