Silver-coated zero-valent iron nanoparticles enhance cancer therapy in mice through lysosome-dependent dual programed cell death pathways: triggering simultaneous apoptosis and autophagy only in cancerous cells†
Abstract
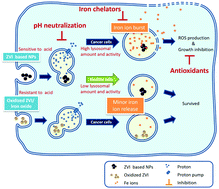
In this study, we demonstrated that zero-valent iron (ZVI), which is widely used to remediate environmental contamination through the production of high-energy reactive oxygen species (ROS), exhibited differential cytotoxicity in cancerous cells and nonmalignant cells. Nanoparticles (NPs) with different shells exhibited distinct potencies against cancerous cells, which depended on their iron-to-oxygen ratios. Silver-coated ZVI NPs (ZVI@Ag) had the highest potency among synthesized ZVI NPs, and they simultaneously exhibited adequate biocompatibility with nonmalignant keratinocytes. The assessment of the intracellular dynamics of iron species revealed that the uptake of ZVI@Ag was similar between cancerous cells and nonmalignant cells during the first 2 h; however, only cancerous cells rapidly converted NPs into iron ions and generated large amounts of intracellular ROS, which was followed by apoptosis and autophagy induction. The aforementioned processes were prevented in the presence of iron ion chelators or by preoxidizing NPs before administration. Neutralization of lysosomal pH effectively reduced ZVI@Ag NP-induced programmed cell death. In the xenograft mouse model, cancer growth was significantly inhibited by a single dose of systematically administered NPs without significant weight loss in animals.


 Please wait while we load your content...
Please wait while we load your content...