The strong electrocaloric effect in molecular ferroelectric ImClO4 with ultrahigh electrocaloric strength†
Abstract
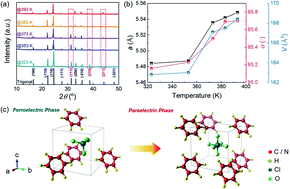
The electrocaloric effect (ECE) provides a new approach to realize environment friendly cooling with high efficiency. Although a giant ECE has been achieved in ferroelectrics, the relatively low EC strength forces conventional EC materials to be operated with very high electric fields, increasing insurmountable obstacles for pushing the ECE to become practical. Here, we reveal an extremely high EC strength (3.6 J mm K−1 kg−1 kV−1 and 0.84 K mm kV−1) in molecular ferroelectric imidazolium perchlorate (ImClO4), which is ∼13 times higher than those of the ferroelectric polymers, and also significantly exceeds those of typical inorganic displacive type ferroelectrics. The superior EC strength is attributable to the unique polarization mechanism arising from the order–disorder behavior of molecular ferroelectric ImClO4 that is completely different from conventional ferroelectrics, which is rationalized by the thermodynamic modeling. As a result, changes of entropy and temperature of 5.4 J kg−1 K−1 and 1.26 K can be attained in ImClO4 with a low electric field of 1.5 kV mm−1. This work suggests a new promising family of ferroelectrics for high-performance solid-state EC cooling.


 Please wait while we load your content...
Please wait while we load your content...