A simple pre-sodiation strategy to improve the performance and energy density of sodium ion batteries with Na4V2(PO4)3 as the cathode material†
Abstract
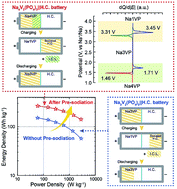
Sodium-ion batteries are considered surrogates for conventional lithium-ion batteries for the commercial sector. One of the most favourable anode materials for sodium-ion batteries is hard carbon. However, its performance is affected by the large irreversible capacity loss (ICL) in the initial charge process. This study proposes the Na4V2(PO4)3 (Na4VP) cathode as a possible solution for matching the hard carbon anode. The process uses a recently developed electrochemical pre-sodiation strategy, where the first step converts Na3V2(PO4)3 (Na3VP) to a Na4VP cathode by a sodiation step. After this, a de-sodiation step of Na4VP is carried out below 2.2 V (vs. Na+/Na) to entirely compensate for the ICL of the anode, and the resulting Na3VP directly acts as the cathode. As a result, the Na4VP‖HC full cell achieves a substantially high specific energy of 265 W h kg−1, which is 76% higher than that of the Na3VP‖HC full cell. Moreover, the Na4VP‖HC full cell shows excellent rate performance (62.32 mA h g−1 at 10C) and cycling stability (about 80.0% capacity retention after 100 cycles at 1C). This work is a precursor to the further study of other battery and hybrid capacitor systems, where pre-sodiation plays an important role in improving their performance.


 Please wait while we load your content...
Please wait while we load your content...