An oxo-verdazyl radical for a symmetrical non-aqueous redox flow battery†
Abstract
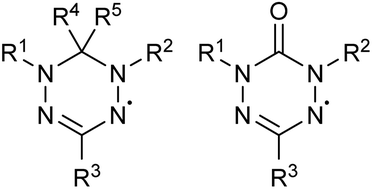
Verdazyl free radical compounds are promising candidates for symmetrical all-organic redox flow batteries (RFBs) due to their redox stability, the ease with which their chemical structure can be varied, and their unique bipolar nature. The present work reports a preliminary screening of a selection of oxo-verdazyl compounds for key redox electrolyte parameters. Of the considered candidates, the 1,5-diphenyl-3-isopropyl-6-oxo-verdazyl radical performed best and is investigated in extensive RFB experiments to compare its electrochemical behavior in cyclic voltammetry (CV) to that within an actual battery. The symmetrical oxo-verdazyl non-aqueous electrolyte RFB provides a mean voltage of 1.42 V and demonstrates good stability as well as high coulombic (>97%) and energy efficiencies over more than 100 charge/discharge cycles. The redox electrolyte is characterized at different stages within a single cycle (‘state of charge’ experiments) independently for each half-cell. To address the specifics of the electrolyte transition to RFB cell setup an ‘in-cell’ CV flow-enabled electrochemical study has been conducted, introduced here as a new step towards standardization of the electrochemical description of RFB electrolytes. The electrochemical performance results highlight oxo-verdazyls as versatile materials for energy applications and indicate great promise for their further development and optimization in the field of RFBs.


 Please wait while we load your content...
Please wait while we load your content...