Post-synthetic modification of ionic liquids using ligand-exchange and redox coordination chemistry†
Abstract
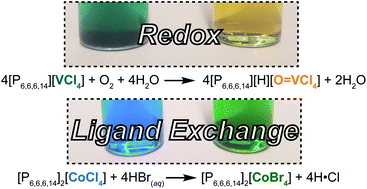
Ionic liquids (ILs) derive their useful properties from molecularly tunable compositions, but methods to diversify anion identities and probe ion speciation remain limited. Here, we demonstrate post-synthetic modification of perhalometallate anions to achieve ionic liquid-to-ionic liquid transformations. Rheological measurements of the metal-containing ILs indicate that minor alterations to anion coordination spheres induce considerable changes to IL viscosities. UV-vis spectra confirm the purities for most ILs, while revealing a surprising cation dependence of perchlorovanadate speciation and supramolecular structure. The intermolecular interactions studied here span a wide range from dispersion to covalent bonding, permitting their impact on IL viscosity to be decoupled and quantified. Together, synthetic strategies from coordination chemistry paired with conventional UV-vis spectroscopy provide a powerful tool for expanding IL compositions and investigating fundamental nanoscale behavior.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...
