Saloplastics as multiresponsive ion exchange reservoirs and catalyst supports†
Abstract
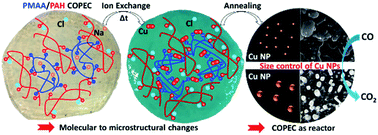
Developing saloplastics composed of Compacted Polyelectrolyte Complexes (COPECs) represents a promising strategy for assembling multifunctional and processable polymer matrices in a simple manner. Here, a comprehensive investigation of the potential application of saloplastics as ion reactors for designing catalysts has been performed. First the propensity of saloplastics to exchange and concentrate ions has been elucidated through investigating the influence of Na+ to Cu2+ cation exchange within COPECs assembled from poly(methacrylic acid) (PMAA) and poly(allylamine hydrochloride) (PAH). The multi-scale responses of PMAA/PAH COPECs upon incubation with CuCl2 solutions at pH 3 and 4.5 were investigated chemically by ATR-FTIR, ICP, XPS, DSC and TGA, morphologically by SEM, and mechanically by strain-to-break measurements. Both the amplitude and the kinetics of the COPEC response were driven by the deprotonation rate of PMAA chains, enabling the formation of bridge complexes with Cu2+ and impacting the saloplastic's composition (water content and polyelectrolytes), structure (emergence of macropores) and mechanical properties. Kinetic-based tuning of the molality of copper ions trapped in PMAA/PAH COPECs was demonstrated, enabling the usage of saloplastics as reactors. This ability allowed controlling the growth of Cu(0) nanoparticles in saloplastics by thermal annealing, ultimately adjusting their catalytic activity toward carbon monoxide (CO) oxidation. This work highlights how the ionic reservoir properties of saloplastics must be accounted for when designing the applications of COPEC-based materials.


 Please wait while we load your content...
Please wait while we load your content...