Suppression of hydrogen evolution at catalytic surfaces in aqueous lithium ion batteries†
Abstract
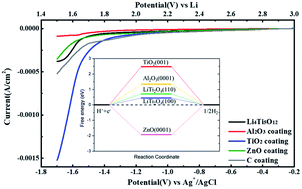
Aqueous lithium ion batteries (ALIBs) have attracted increasing attention due to their excellent safety profile. The water-in-salt electrolyte (WiSE) has enabled a wider voltage window (3.0 V) through the formation of an solid–electrolyte–interphase (SEI) on the anode. However, the cathodic limit of the WiSE and its derivatives cannot effectively support the desired energy-dense anodes, such as Li4Ti5O12 (LTO). At the anode, the hydrogen evolution reaction (HER) is the main parasitic process that competes with the desired lithiation process therein. We investigated the catalytic activity of different coating layers and postulated the selection criterion for the surface layers. We demonstrated that Al2O3 had a surface that effectively suppressed the HER and enabled the cycling of the LTO anode in the WiSE, thereby delivering a capacity of 145 mA h g−1. Such understanding provides important guidelines for designing electrolytes and interphases for aqueous battery chemistries.


 Please wait while we load your content...
Please wait while we load your content...
