Structural evolution of disordered LiCo1/3Fe1/3Mn1/3PO4 in lithium batteries uncovered†
Abstract
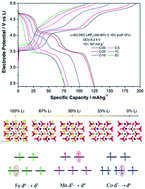
In this study we address the Li-ion de-insertion/insertion mechanisms from/into the lattice of the mixed olivine LiCo1/3Fe1/3Mn1/3PO4 (LCFMP). This mechanism is driven by a subtle interplay of structural, electronic and thermodynamic features. We aim at dissecting this complex landscape that is tightly connected to the long-term electrochemical performance of this material as a positive electrode in lithium-ion cells. To this end, we report advanced structural characterization, based on ex situ synchrotronradiation diffraction on samples at different lithium contents. We couple this analysis with first-principles simulations, for a direct vis-à-vis comparison. Our results show that (1) the mixing of the three transition-metal (TM) cations in the olivine lattice leads to a solid solution, providing the olivine lattice with the necessary flexibility to retain its single-phase structure during cell operation; (2) the electronic features of the three TMs are responsible for the observed electrochemical performance; (3) the de-lithiation of the olivine lattice is a thermodynamically driven process. Last but not least, our integrated experimental and theoretical results reveal the subtle features behind the formation of antisite defects that selectively involve Li–Co couples. In conclusion, our study provides the necessary scientific foundations to understand the structure–property–function relationships in LCFMP olivines, paving the way for further development and optimization of this material for application in Li-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...