Towards the understanding of acetonitrile suppressing salt precipitation mechanism in a water-in-salt electrolyte for low-temperature supercapacitors†
Abstract
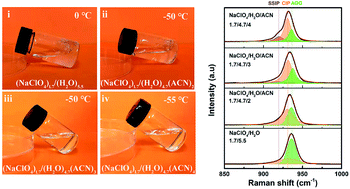
Although water-in-salt (WIS) electrolytes have significantly extended the voltage window of aqueous batteries and supercapacitors, the inevitable precipitation of highly concentrated salts at low temperatures leads to performance degradation and even failure of devices. The introduction of an organic co-solvent can effectively overcome this crucial drawback; however, the underlying mechanism remains unclear. Herein, we demonstrate the study of acetonitrile (ACN) suppressing salt precipitation mechanism in NaClO4-based WIS electrolytes through combining theoretical simulation and experimental analyses. ACN molecules strongly coordinate with Na+ ions to change the solvation structure of the cation–anion from aggregates to contact ion pairs and/or solvent separated ion pairs, weakening the interaction between anions and cations, thereby suppressing the precipitation of NaClO4. Furthermore, using the hybrid electrolyte, a built carbon-based supercapacitor achieves a voltage window of 2.3 V. Impressively, it also retains 86.5% of the room-temperature capacitance and exhibits excellent rate capability and temperature resistance at an ultra-low temperature of −50 °C. This work provides guidance for non-inert co-solvents suppressing salt precipitation, which helps to obtain desired electrolytes for reliable low-temperature supercapacitors.


 Please wait while we load your content...
Please wait while we load your content...