The favourable thermodynamic properties of Fe-doped CaMnO3 for thermochemical heat storage†
Abstract
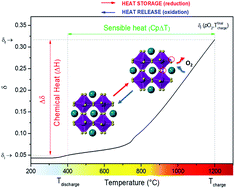
The CaMnO3 oxide can reversibly release oxygen over a relatively wide range of temperatures and oxygen partial pressures (pO2) and is thus a promising candidate for thermochemical heat storage in Concentrated Solar Power (CSP) plants. Moreover, it is composed of earth-abundant, inexpensive and non-toxic elements and exhibits a high-energy storage density, which are desirable characteristics for decreasing the deployment costs of the system. However, it undergoes decomposition at pO2 ≤ 0.008 atm and temperature ≥ 1100 °C. Here the possibility of overcoming this limitation and extending the operating temperature range by B-site doping with Fe (CaFexMn1−xO3−δ0) is explored. Two doping levels are investigated, x = 0.1 and 0.3. The enthalpy of reduction was determined from a measurement of continuous equilibrium non-stoichiometry curves (δ, T) at several pO2, enabling an evaluation of the heat storage capacity with high accuracy over widely ranging oxygen non-stoichiometry. Introduction of 0.1 Fe (CaFe0.1Mn0.9O3−δ0) prevented CaMnO3 decomposition up to 1200 °C at pO2 = 0.008 atm, thus widening the operating temperature range and the oxygen reduction extent. The increase in the accessible nonstoichiometry translates into an increase in the heat storage capacity (QM (kJ molABO3−1)) from ∼272 kJ kgABO3−1 in CaMnO3 to ∼344 kJ kgABO3−1 in CaFe0.1Mn0.9O3−δ0. While even larger changes in oxygen content were accessible in CaFe0.3Mn0.7O3−δ0, the oxidation state changes are accompanied by a lower enthalpy of reduction, resulting in a diminished heat storage capacity of ∼221 kJ kgABO3−1.


 Please wait while we load your content...
Please wait while we load your content...