Exceeding the volcano relationship in oxygen reduction/evolution reactions using single-atom-based catalysts with dual-active-sites†
Abstract
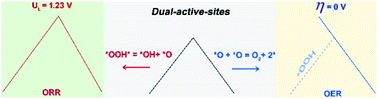
Finding cost-effective catalysts to drive oxygen reduction/evolution reactions (ORR/OER) is a highly attractive goal. Most catalysts follow a volcano relationship of performance, making it difficult to search thoroughly enough among the huge number of possible structures to reach the volcano apex. Using first-principles simulations, we demonstrated that the design of single-atom-based catalysts (SACs) incorporating dual-active-sites breaks the universal scaling relationship between *OOH and *OH adsorption, leading to performances superior to those constrained to follow the volcano plot. Both a linear OER activity trend that reaches an ideal 0 V overpotential and a new linear scaling relation (free energy difference ΔGOOH = ΔGOH + 2.41 eV) that crosses the region of optimal limiting potentials in the volcano plot of the ORR are associated with our dual-active-site designs. This novel strategy of breaking the volcano dependence with dual-active-sites in SACs may promote the development of efficient electrocatalysts for the ORR/OER and other chemical reactions.


 Please wait while we load your content...
Please wait while we load your content...