Understanding the mechanism of byproduct formation with in operando synchrotron techniques and its effects on the electrochemical performance of VO2(B) nanoflakes in aqueous rechargeable zinc batteries†
Abstract
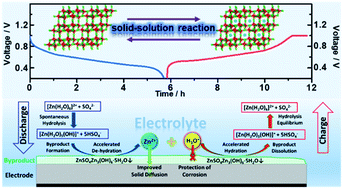
Monoclinic VO2(B) nanoflakes prepared by a hydrothermal method displayed superior electrochemical performance in 1 M ZnSO4 electrolyte. The reaction mechanisms of VO2(B) and the essential causes of byproduct formation in aqueous rechargeable zinc batteries (ARZBs) were comprehensively studied by electrochemical measurements combined with in operando synchrotron techniques and first-principles calculations. During the electrochemical processes, the electrode underwent a reversible solid-solution reaction between VO2(B) and Zn0.44VO2 with the simultaneous formation/decomposition of the (Zn(OH)2)3(ZnSO4)·5H2O byproduct. Importantly, the formation of the byproduct was attributed to [Zn(H2O)6]2+ dehydration, where the byproduct could protect the electrode material from the corrosion of H3O+ and facilitate the dehydration process of Zn2+ on the electrode–electrolyte interface. The byproducts could facilitate the migration of Zn2+ on the electrode surface due to their three-dimensional pathways. In addition, the electrochemical performance of VO2(B) and the byproduct in ZnSO4 electrolyte were compared with those in Zn(CF3SO3)2 and Zn(NO3)2. An appropriate electrolyte (1 M Zn(CF3SO3)2) to form a byproduct with largely expanded ionic pathways was proven to further improve the electrochemical performance of VO2(B). This work not only provides a deep understanding of the Zn2+ storage mechanism in VO2(B) but also establishes a clear relationship between the byproducts and electrochemical performance of vanadium-based electrode materials in ARZBs.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...