Mechanistic understanding of pore evolution enables high performance mesoporous silicon production for lithium-ion batteries†
Abstract
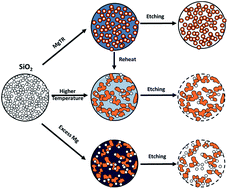
The cycling of silicon anodes within a lithium-ion battery (LIB) leads to degradation and capacity fade due to the 280% volume change of silicon. Many methods of silicon synthesis have been explored to produce nanostructures which can withstand this change in volume. Magnesiothermic Reduction (MgTR) shows significant promise over other syntheses in scalability, economic and environmental aspects for producing porous silicon nanostructures. The problem with MgTR is a lack of understanding regarding the pore evolution of porous silicon based on reduction parameters and precursor materials, which in turn limits predictive design for desired applications. Here we show that the pore structure of porous silicon is strongly related to the interconnectivity of silicon crystallites. We show that MgTR is a thermodynamically driven equilibrium which determines the purity of the silicon product. Higher temperatures also cause sintering of silicon nanocrystallites. We show that it is the interconnectivity of these crystallites that determines the pore size and distribution within porous silicon. These findings apply to a wide variety of porous silica precursors and we show that this mechanism is true for the introduction of pores into nonporous quartz after MgTR. Furthermore, we show that by exploiting this mechanism, mesoporous silicon can be produced which has excellent promise for LIB applications with a capacity of 2170 mA h g−1 after 100 cycles. The findings herein can be taken forward to design optimal materials for LIB applications. These results strongly support the potential for reduction in silicon costs for LIBs in both economic and environmental terms as well as for a reverse engineering approach to design specific porous silicon for desired applications even beyond LIBs.


 Please wait while we load your content...
Please wait while we load your content...