Diffusiophoresis: from dilute to concentrated electrolytes
Abstract
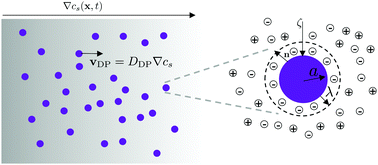
Electrolytic diffusiophoresis is the movement of colloidal particles in response to a concentration gradient of an electrolyte. The diffusiophoretic velocity vDP is typically predicted through the relation vDP = DDP ∇log cs, where DDP is the diffusiophoretic mobility and cs is the concentration of the electrolyte. The logarithmic dependence of vDP on cs may suggest that the strength of diffusiophoretic motion is insensitive to the magnitude of the electrolyte concentration. In this article, we emphasize that DDP is intimately coupled with cs for all electrolyte concentrations. For dilute electrolytes, the finite double layer thickness effects are significant such that DDP decreases with a decrease in cs. In contrast, for concentrated electrolytes, charge screening could result in a decrease in DDP with an increase in cs. Therefore, we predict a maximum in DDP with cs for moderate electrolyte concentrations. We also show that for typical colloids and electrolytes 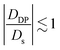 , where Ds is the solute ambipolar diffusivity. To validate our model, we conduct microfluidic experiments with a wide range of electrolyte concentrations. The experimental data also reveals a maximum in DDP with cs, in agreement with our predictions. Our results have important implications in the broad areas of electrokinetics, lab-on-a-chip, active colloidal transport and biophysics.
, where Ds is the solute ambipolar diffusivity. To validate our model, we conduct microfluidic experiments with a wide range of electrolyte concentrations. The experimental data also reveals a maximum in DDP with cs, in agreement with our predictions. Our results have important implications in the broad areas of electrokinetics, lab-on-a-chip, active colloidal transport and biophysics.
- This article is part of the themed collection: Soft Matter Lectureship Winners


 Please wait while we load your content...
Please wait while we load your content...