High antisite defect concentrations in hard-sphere colloidal Laves phases†
Abstract
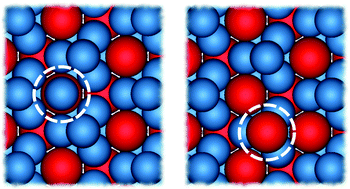
Binary mixtures of hard spheres can spontaneously self-assemble into binary crystals. Computer simulations have been especially useful in mapping out the phase behaviour of these mixtures, under the assumption that the stoichiometry of the binary crystal is ideal. Here we show that for a size ratio of q = 0.82 this assumption is not valid near the coexistence region between the fluid and the stable binary crystal, the MgZn2 Laves phase. Instead we find a surprisingly high number of antisite defects: up to 2% of the large spheres are replaced by small spheres in equilibrium. We demonstrate that the defect concentration can be estimated using simple approximations, providing an easy way to identify systems where antisite defects play an important role. Our results shed new light on the self-assembly of colloidal Laves phases, and demonstrate the importance of antisite defects in binary crystals.


 Please wait while we load your content...
Please wait while we load your content...