The tripeptide GHG as an unexpected hydrogelator triggered by imidazole deprotonation†
Abstract
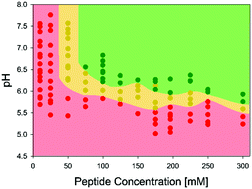
The tripeptide glycyl-histidyl-glycine (GHG) self-assembles into long, crystalline fibrils forming a strong hydrogel (G′ ∼ 50 kPa) above a critical concentration of 40 mM upon the deprotonation of its imidazole group. Spectroscopic data reveal a mixture of helically twisted β-sheets and monomers to coexist in the gel phase.


 Please wait while we load your content...
Please wait while we load your content...
