Boosting the oxygen evolution activity in non-stoichiometric praseodymium ferrite-based perovskites by A site substitution for alkaline electrolyser anodes†
Abstract
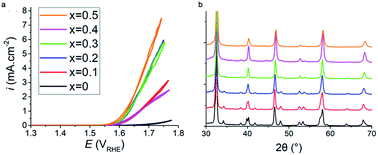
Sustainable fossil fuel free systems are crucial for tackling climate change in the global energy market, and the identification and understanding of catalysts needed to build these systems plays a vital role in their development. ABO3−δ perovskite oxides have been observed to be potential replacement materials for the high-performing, but low ionic conducting and economically unfavourable Pt and IrO2 water splitting catalysts. In this work increased addition of Sr2+ aliovalent dopant ions into the crystal lattice of Pr1−xSrxFeO3−δ perovskites via A site substitution was seen to drastically improve the electrocatalytic activity of the oxygen evolution reaction (OER) in alkaline environments. The undoped PrFeO3−δ catalyst was not catalytically active up to 1.70 V against the reversible hydrogen electrode (RHE), whilst an onset potential of 1.62 V was observed for x = 0.5. Increased strontium content in Pr1−xSrxFeO3−δ was found to cause a reduction in the lattice parameters and crystal volume whilst retaining the orthorhombic Pbnm space group throughout all dopant levels, analysed using the Rietveld method. However, it was noted that the orthorhombic distortion was reduced as more Sr2+ replaced Pr3+. The mechanism for the increased electrocatalytic activity with increased strontium is due to the increasing concentration of oxygen vacancy (δ), leading to increased catalyst site availability, and the increased average oxidation state of Fe cations, consistent with the iodometric titration results. This results in shifting the average d shell eg electron filling further towards unity. X-ray photoelectron spectrum of the O 1s core level also shows the presence of lattice oxide and surface hydroxide/carbonate. This work shows promise in that using the more abundant and more economically friendly material of strontium allows for improved OER catalytic activity in otherwise inactive perovskite catalyst oxides.


 Please wait while we load your content...
Please wait while we load your content...