Methylbismuth: an organometallic bismuthinidene biradical†
Abstract
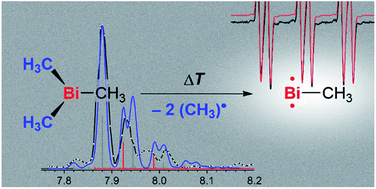
We report the generation, spectroscopic characterization, and computational analysis of the first free (non-stabilized) organometallic bismuthinidene, BiMe. The title compound was generated in situ from BiMe3 by controlled homolytic Bi–C bond cleavage in the gas phase. Its electronic structure was characterized by a combination of photoion mass-selected threshold photoelectron spectroscopy and DFT as well as multi-reference computations. A triplet ground state was identified and an ionization energy (IE) of 7.88 eV was experimentally determined. Methyl abstraction from BiMe3 to give [BiMe2]• is a key step in the generation of BiMe. We reaveal a bond dissociation energy of 210 ± 7 kJ mol−1, which is substantially higher than the previously accepted value. Nevertheless, the homolytic cleavage of Me–BiMe2 bonds could be achieved at moderate temperatures (60–120 °C) in the condensed phase, suggesting that [BiMe2]• and BiMe are accessible as reactive intermediates under these conditions.
- This article is part of the themed collections: Celebrating 10 years of Chemical Science and 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...