Transparent and luminescent glasses of gold thiolate coordination polymers†
Abstract
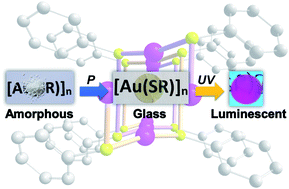
Obtaining transparent glasses made of functional coordination polymers (CPs) represents a tremendous opportunity for optical applications. In this context, the first transparent and red-emissive glasses of gold thiolate CPs have been obtained by simply applying mechanical pressure to amorphous powders of CPs. The three gold-based CP glasses are composed of either thiophenolate [Au(SPh)]n, phenylmethanethiolate [Au(SMePh)]n or phenylethanethiolate [Au(SEtPh)]n. The presence of a longer alkyl chain between the thiolate and the phenyl ring led to the formation of glass with higher transparency. The glass transitions, measured by thermomechanical analysis (TMA), occurred at lower temperature for CPs with longer alkyl chains. In addition, all three gold thiolate glasses exhibit red emission at 93 K and one of them, [Au(SMePh)]n, remains luminescent even at room temperature. An in-depth structural study of the amorphous gold thiolates by XRD, PDF and EXAFS analysis showed that they are formed of disordered doubly interpenetrated helical chains. These d10 metal-based compounds represent the first examples of transparent and luminescent CP glasses.


 Please wait while we load your content...
Please wait while we load your content...