Direct catalytic asymmetric and anti-selective vinylogous addition of butenolides to chromones†
Abstract
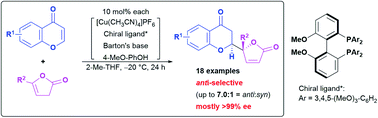
An anti-selective catalytic asymmetric Michael-type vinylogous addition of β,γ-butenolides to chromones was developed. The catalyst system developed herein is characterized by tuning of the steric and electronic effects using a proper Biphep-type chiral ligand to invert the diastereoselection, and improvement of the catalyst turnover by a coordinative phenolic additive. The catalytic protocol renders potentially biologically active natural product analogs accessible in good yield with moderate diastereoselectivity and high enantiomeric purity, mostly greater than 99% ee.


 Please wait while we load your content...
Please wait while we load your content...