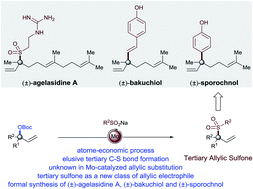
Regioselective molybdenum-catalyzed allylic substitution of tertiary allylic electrophiles: methodology development and applications†
Abstract
The first molybdenum-catalyzed allylic sulfonylation of tertiary allylic electrophiles is described. The method employs a readily accessible catalyst (Mo(CO)6/2,2′-bipyridine, both are commercially available) and represents the first example of the use of a group 6 transition metal-catalyst for allylic sulfonylation of substituted tertiary allylic electrophiles to form carbon–sulfur bonds. This atom economic and operationally simple methodology is characterized by its relatively mild conditions, wide substrate scope, and excellent regioselectivity profile, thus unlocking a new platform to forge sulfone moieties, even in the context of late-stage functionalization and providing ample opportunities for further derivatization through traditional Suzuki cross-coupling reactions.


 Please wait while we load your content...
Please wait while we load your content...