Thermodynamic consequences of Tyr to Trp mutations in the cation–π-mediated binding of trimethyllysine by the HP1 chromodomain†
Abstract
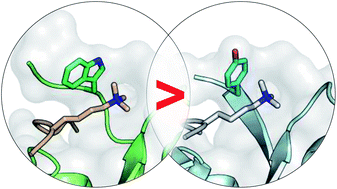
Evolution has converged on cation–π interactions for recognition of quaternary alkyl ammonium groups such as trimethyllysine (Kme3). While computational modelling indicates that Trp provides the strongest cation–π interaction of the native aromatic amino acids, there is limited corroborative data from measurements within proteins. Herein we investigate a Tyr to Trp mutation in the binding pocket of the HP1 chromodomain, a reader protein that recognizes Kme3. Binding studies demonstrate that the Trp-mediated cation–π interaction is about −5 kcal mol−1 stronger, and the Y24W crystal structure shows that the mutation is not perturbing. Quantum mechanical calculations indicate that greater enthalpic binding is predominantly due to increased cation–π interactions. NMR studies indicate that differences in the unbound state of the Y24W mutation lead to enthalpy–entropy compensation. These results provide direct experimental quantification of Trp versus Tyr in a cation–π interaction and afford insight into the conservation of aromatic cage residues in Kme3 reader domains.


 Please wait while we load your content...
Please wait while we load your content...