Accurate chiral pattern recognition for amines from just a single chemosensor†
Abstract
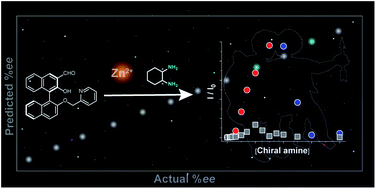
The current work proposes a novel determination method for enantiomeric excess (ee) in (mono- and di-) amines using molecular self-assembly. A pyridine-attached binaphthyl derivative ((R)-1) exhibits fluorescence responses based on imine formation between the aldehyde group of (R)-1 and target chiral amines (i.e. cyclohexane diamine (CHDA), 2-amino-1,2-diphenylethanol (ADPE), 1,2-diphenylethylenediamine (DPDA), 1-amino-2-indanol (AID), and leucinol) in the presence of zinc(II) ions (Zn2+). Because of the multi-optical responses which are derived from the variation of chiral complexes, pattern recognition-based discrimination (i.e. linear discriminant analysis (LDA)) has been achieved for five types of enantiomeric pairs of amines. Possessing such a discrimination capability in combination with data processing (LDA and an artificial neural network) allows accurate determination (prediction error < 1.8%) of the % ee of individual targets such as CHDA which is one of the main components of pharmaceutical drugs. The simple molecular self-assembled system enabled simultaneous multi-chiral discrimination and % ee determination of unknown samples.
- This article is part of the themed collections: 2020 Chemical Science HOT Article Collection and The Mechanics of Supramolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...