Selective radical depolymerization of cellulose to glucose induced by high frequency ultrasound†
Abstract
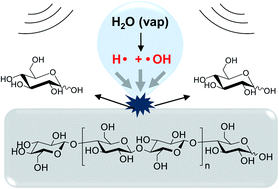
The depolymerization of cellulose to glucose is a challenging reaction and often constitutes a scientific obstacle in the synthesis of downstream bio-based products. Here, we show that cellulose can be selectively depolymerized to glucose by ultrasonic irradiation in water at a high frequency (525 kHz). The concept of this work is based on the generation of H˙ and ˙OH radicals, formed by homolytic dissociation of water inside the cavitation bubbles, which induce the cleavage of the glycosidic bonds. The transfer of radicals on the cellulose particle surfaces prevents the side degradation of released glucose into the bulk solution, allowing maintaining the selectivity to glucose close to 100%. This work is distinguished from previous technologies in that (i) no catalyst is needed, (ii) no external source of heating is required, and (iii) the complete depolymerization of cellulose is achieved in a selective fashion. The addition of specific radical scavengers coupled to different gaseous atmospheres and ˙OH radical dosimetry experiments suggested that H˙ radicals are more likely to be responsible for the depolymerisation of cellulose.


 Please wait while we load your content...
Please wait while we load your content...