Lipshutz-type bis(amido)argentates for directed ortho argentation†
Abstract
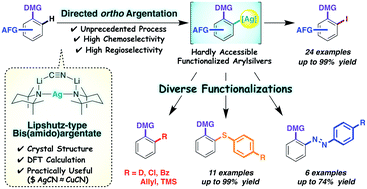
Bis(amido)argentate (TMP)2Ag(CN)Li2 (3, TMP-Ag-ate; TMP = 2,2,6,6-tetramethylpiperidido) was designed as a tool for chemoselective aromatic functionalization via unprecedented directed ortho argentation (DoAg). X-Ray crystallographic analysis showed that 3 takes a structure analogous to that of the corresponding Lipshutz cuprate. DoAg with this TMP-Ag-ate afforded multifunctional aromatics in high yields in processes that exhibited high chemoselectivity and compatibility with a wide range of functional groups. These included organometallics- and transition metal-susceptible substituents such as methyl ester, aldehyde, vinyl, iodo, (trifluoromethanesulfonyl)oxy and nitro groups. The arylargentates displayed good reactivity with various electrophiles. Chalcogen (S, Se, and Te) installation and azo coupling reactions also proceeded efficiently.
- This article is part of the themed collection: Editor’s Choice – Ning Jiao


 Please wait while we load your content...
Please wait while we load your content...