Palladium-catalyzed intermolecular transthioetherification of aryl halides with thioethers and thioesters†
Abstract
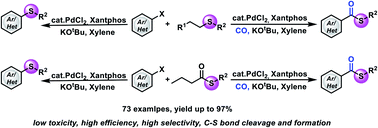
Functional group transfer reactions are an important synthetic tool in modern organic synthesis. Herein, we developed a new palladium-catalyzed intermolecular transthioetherification reaction of aryl halides with thioethers and thioesters. The synthetic utility and practicality of this catalytic protocol are demonstrated in a wide range of successful transformations (>70 examples). This catalytic protocol is applicable in carbonylative coupling processes as well, and the first example of carbonylative methylthioesterification of aryl halides has been achieved. Notably, this work also provides an approach to using natural products, such as methionine and selenomethionine, as the functional group sources.


 Please wait while we load your content...
Please wait while we load your content...