Enzymes in a golden cage†
Abstract
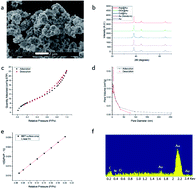
We describe a general method for the entrapment of enzymes within bulk metallic gold. This is a new approach for the immobilization of enzymes on metals, which is commonly carried out by 2D adsorption or covalent biding, that is, the enzyme is in contact with the metal at a specific contact zone of the enzyme, while most of the rest of it remains exposed to the environment. The 3D metallic encaging of the enzymes is quite different: the enzyme is in contact with the metallic cage walls all around it and is well protected inside. The porous nature of the metallic matrix enables substrate molecules to diffuse inside, reach the active site, and let product molecules diffuse out. The generality of the approach was proven by the successful entrapment of five enzymes representing different classes and different bio- and medical applications: L-asparaginase (Asp), collagenase, horseradish peroxidase (HRP), laccase and glucose oxidase (GOx). GOx–gold conjugates have been of particular interest in the literature. The main challenge we had to solve was how to keep the enzyme active in the process of gold-synthesis from its cation – this required careful tailoring of reaction conditions, which are detailed in the paper. The gold entrapped enzymes gain thermal stability and protectability against harsh conditions. For instance, we could keep Asp alive at the extreme pH of 13, which normally kills the enzyme instantly. The entrapped enzymes obey the Michaelis–Menten kinetics, and activation energies were determined. Good recyclability for eight cycles was found. Multi-enzymatic reactions by combinations of the off-the-shelf bioactive enzyme@gold powders are possible, as demonstrated for the classical detection of GOx activity with HRP. Detailed material characterization and proposed mechanisms for the 3D protectability of the enzymes are provided. The new enzyme immobilization method is of wide potential uses in medicine, biotechnology, bio-fuel cells and enzymatic (electro)sensing applications.


 Please wait while we load your content...
Please wait while we load your content...