Palladium catalysed C–H arylation of pyrenes: access to a new class of exfoliating agents for water-based graphene dispersions†
Abstract
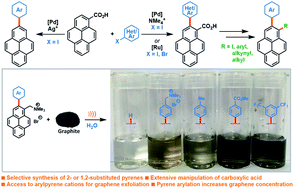
A new and diverse family of pyrene derivatives was synthesised via palladium-catalysed C–H ortho-arylation of pyrene-1-carboxylic acid. The strategy affords easy access to a broad scope of 2-substituted and 1,2-disubstituted pyrenes. The C1-substituent can be easily transformed into carboxylic acid, iodide, alkynyl, aryl or alkyl functionalities. This approach gives access to arylated pyrene ammonium salts, which outperformed their non-arylated parent compound during aqueous Liquid Phase Exfoliation (LPE) of graphite and compare favourably to state-of-the-art sodium pyrene-1-sulfonate PS1. This allowed the production of concentrated and stable suspensions of graphene flakes in water.


 Please wait while we load your content...
Please wait while we load your content...